Can a Mixture Be Separated by Physical Means
Summary
Students larn how to classify materials as mixtures, elements or compounds and identify the backdrop of each type. The concept of separation of mixtures is also introduced since nearly every chemical element or compound is plant naturally in an impure state such as a mixture of 2 or more substances, and it is common that chemical engineers utilize separation techniques to separate mixtures into their individual components. For case, the separation of crude oil into purified hydrocarbons such as natural gas, gasoline, diesel fuel, jet fuel and/or lubricants.Engineering Connexion
Almost all materials in the universe are constitute naturally in impure states such equally mixtures of two or more substances. In chemistry and chemical engineering, a separation process is commonly used to transform a mixture of substances into two or more distinct materials. The separated products might differ in chemic properties or some physical belongings, such as size or crystal modification or other separation into different components. I example of separation application is crude oil, which is a mixture of various hydrocarbons. While valuable in this natural form, demand is greater for the various purified hydrocarbons such as natural gases, gasoline, diesel, jet fuel, lubricating oils, asphalt, etc. Chemical plants usually have from xl% to seventy% of both capital and operating costs in separations.[i] Since separations are ubiquitous in chemic plants and petroleum refineries, chemical engineers must be familiar with a variety of separation methods.
Learning Objectives
Subsequently this lesson, students should exist able to:
- Define chemical element, mixture and compound.
- Explain the differences between pure substances and mixtures.
- Explicate the characteristics of the mixtures.
- Give some examples of elements, mixtures and compounds.
- Define the homogenious and heterogeneous mixtures and requite some examples.
- Explain in full general how mixtures can be separated.
- Name some separation techniques.
- Explain how chemic engineers apply these separation methods to purify diverse hydrocarbons such as natural gases, gasoline, diesel, jet fuel, lubricating oils, cobblestone, etc., from raw crude oil.
Educational Standards Each TeachEngineering lesson or activity is correlated to 1 or more K-12 science, technology, engineering science or math (Stalk) educational standards.
All 100,000+ K-12 Stalk standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.yard., by land; inside source by blazon; e.g., science or mathematics; inside type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to 1 or more K-12 science, technology, engineering science or math (Stalk) educational standards.
All 100,000+ K-12 Stalk standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.yard., by land; inside source by blazon; e.g., science or mathematics; inside type by subtype, then by grade, etc.
International Applied science and Applied science Educators Clan - Technology
- Chemical technologies provide a means for humans to alter or modify materials and to produce chemic products. (Grades ix - 12) More Details
View aligned curriculum
Do you agree with this alignment? Thank you for your feedback!
- Materials have different qualities and may be classified as natural, synthetic, or mixed. (Grades 9 - 12) More Details
View aligned curriculum
Practice yous agree with this alignment? Thanks for your feedback!
State Standards
Suggest an alignment not listed aboveWorksheets and Attachments
Visit [world wide web.teachengineering.org/lessons/view/uoh_sep_mixtures_less1] to print or download.More Curriculum Like This
High School Activeness 
Eat Iron?!
To proceeds an understanding of mixtures and the concept of separation of mixtures, students utilise strong magnets to detect the element of iron in iron-fortified breakfast cereal flakes. Through this activity, they meet how the iron component of this heterogeneous mixture (cereal) retains its properties and ...

High School Activity 
Element, Mixture, Compound
Students gain a better agreement of the different types of materials as pure substances and mixtures and learn to distinguish between homogeneous and heterogeneous mixtures past discussing an array of example materials they employ and come across in their daily lives.

Upper Simple Lesson 
Properties of Mixtures vs. Solutions: Mix It Up!
This lesson program introduces students to the properties of mixtures and solutions. Information technology includes instructor instructions for a class demonstration that gives students the chance to compare and contrast the physical characteristics of some simple mixtures and solutions.

Upper Simple Lesson 
Understanding Elements
Students examine the periodic tabular array and the backdrop of elements. They learn the basic definition of an element and the eighteen elements that etch virtually of the matter in the universe. The periodic tabular array is described as one method of organization for the elements.

Introduction/Motivation
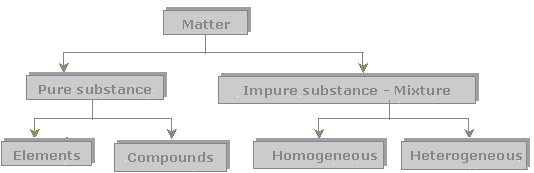
Pure Substances vs. Mixtures
Everything effectually u.s. is made of thing. Affair tin be classified in to two wide categories: pure substances and mixtures.
Lavoisier, a French chemist, classified pure substances into elements and compounds on the basis of quantitative studies. He showed that when we rut mercuric oxide it changes into mercury and oxygen.[ii]
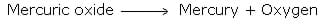
Mercuric oxide is a chemical compound considering it decomposes into simpler substances, whereas mercury and oxygen cannot exist farther decomposed into anything simpler equally they are elements.
Pure Substances
i. Elements
All matter is composed of elements that are fundamental substances that cannot be broken down by chemical means. Element is defined as a substance that tin not be further reduced equally to simpler substances by ordinary processes. Another definition of element: a material that is composed of merely ane type of atom. The elements hydrogen, carbon, nitrogen and oxygen are the elements that make upwardly virtually living organisms. Some other elements found in living organisms are magnesium, calcium, phosphorus, sodium and potassium.
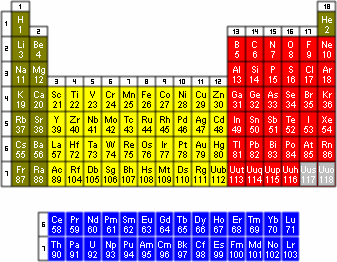
Of the 114 known elements, 92 occur in nature. By the late 1800s many elements had already been discovered. Dmitri Mendeleev, a Russian chemist, proposed an arrangement of known elements based on their diminutive masses. The modern arrangement of the elements, the periodic tabular array of elements, is a tabular display of the known chemical elements (see Figure i) in which they are bundled according to their diminutive numbers. The elements are bundled by electron construction and then that many chemic properties vary systematically beyond the table. Each element is listed by its atomic number and chemical symbol.
2. Compounds
A compound is a pure substance composed of two or more different atoms chemically bonded to one another. That ways that information technology can not exist separated into its constituents by mechanical or concrete means and only tin exist destroyed by chemical ways. For example if we bring a magnet most a sample of iron sulphide, the iron present in the iron sulphide can not be separated.
Properties of a compound differ entirely from those of its constituent elements. Water is equanimous of hydrogen and oxygen. Still, the properties of hydrogen and oxygen (both gases) are unlike from water (liquid). Hydrogen is combustible, oxygen is a supporter of combustion whereas water (made upward of both hydrogen and oxygen) puts out a flame.
Energy changes are involved in the formation of a chemical compound. For instance, iron and sulphur react only when estrus is supplied. The constituent elements in a chemical compound are in a fixed proportion by weight. In water, hydrogen and oxygen are present in a stock-still ratio of 1:viii by weight.
A compound is a homogeneous substance. That is, it is same throughout in properties and composition. Compounds also have fixed melting and humid points.
Mixtures
A mixture is a material containing 2 or more elements or compounds that are in shut contact and are mixed in any proportion. For instance, air, sea water, crude oil, etc. The constituents of a mixture can be separated by concrete means like filtration, evaporation, sublimation and magnetic separation. In the preparation of a mixture, energy is neither evolved nor absorbed. A mixture has no definite melting and boiling points. The constituents of a mixture retain their original prepare of properties. For example, sulphur dissolves in carbon disulphide and a magnet attracts iron filings. To assist illustrate mixtures and dissimilar types refer to the associated activity Element, Mixture, Compound for students to hash out materials they use in their daily lives to gain a better understanding of pure substances vs. mixtures, and homogeneous vs. heterogeneous mixtures.
Examples of mixtures:
- Solid in liquid: Sugar and coffee
- Liquid in liquid: Water and alcohol
- Gas in liquid: Soda
- Gas in solid: Air entrapped in soil
- Gas in gas: Air containing hydrogen, oxygen, nitrogen, carbon dioxide, etc.
- Solid in solid: Metal alloys
1. Homogeneous Mixtures
The prefix "homo" indicates sameness. A homogeneous mixture has the same uniform appearance and composition throughout its mass. For instance, sugar or table salt dissolved in h2o, alcohol in water, etc.
2. Heterogeneous Mixtures
The prefix "hetero" indicates difference. A heterogeneous mixture consists of visibly different substances or phases. The iii phases or states of matter are gas, liquid and solid. A heterogeneous mixture does not have a compatible composition throughout its mass.
Separation of Components of Mixtures
Most materials institute in nature are in the class of mixtures. In engineering, a separation procedure is used to transform a mixture into 2 or more singled-out products. This is washed by considering that unlike components of the mixture may take dissimilar properties such as:
- size
- density
- solubility
- electric accuse
- boiling point
Depending on the raw mixture, various processes tin can exist employed to split the mixtures. Often, two or more of these processes must exist used in combination to obtain the desired separation. In addition to chemical processes, mechanical processes are sometimes applied. In the instance of crude oil, 1 upstream distillation operation feeds its two or more product streams into multiple downstream distillation operations to further separate the raw crude, and so on, until final products are purified.
Example separation techniques for mixtures:
- Filtration is used for the separation of solids from fluids (liquids or gases) by interposing a medium through which only the fluid tin pass.
- Distillation for mixtures of liquids with dissimilar boiling points.
- Chromatography separates dissolved substances by different interaction with (that is, travel through) a fabric.
- Centrifugation and cyclonic separation, separates based on density differences.
- Drying, removes liquid from a solid by vaporization.
- Magnet separation technique uses magnet to separate iron particles from a mixture. (Refer to the associated activeness Eat Fe?!! for a fun experiment to show students how the atomic number 26 component of this heterogeneous mixture (cereal) retains its properties and can thus be separated by physical ways.)
Chemical engineers use these separation techniques to purify naturally constitute substances or isolate them from other substances. For example, crude oil, likewise called petroleum, is a complex mixture of carbon and hydrogen (hydrocarbons) that exists as a liquid in the Globe's chaff. Chemical engineers use diverse distillation methods to purify various hydrocarbons such as natural gases, gasoline, diesel, jet fuel, lubricating oils, asphalt, etc., from the raw crude oil. Water purification is some other good instance of application of separation techniques.
Lesson Groundwork and Concepts for Teachers
Several types of mixtures exist:
- Solutions are mixtures fabricated by mixing a solute and a solvent, like salt in h2o. The solute is the substance that dissolves. The solvent is the substance that does the dissolving. Solutions are homogeneous.
- Suspensions are heterogeneous mixtures of a solid and a liquid in which the solid does non dissolve, similar sand in water. Suspensions settle when left continuing undisturbed.
- Emulsions are a special blazon of interruption. This mixture consists of two liquids that do not mix, like oil and water. Since the liquids do non mix, emulsions are heterogeneous. Emulsions settle into layers when they are left standing undisturbed.
- Colloidal Dispersions are mixtures with characteristics partway between a solution and a pause, like mayonnaise. Colloidal dispersions may appear homogeneous just are actually heterogeneous. Colloidal dispersions do non settle when left standing undisturbed for a catamenia of fourth dimension.
Associated Activities
- Element, Mixture, Compound - Students discuss materials they use in their daily lives to gain a improve understanding of pure substances vs. mixtures, and homogeneous vs. heterogeneous mixtures.
Watch this action on YouTube
- Consume Iron?! - Students use strong magnets to observe the element of iron in atomic number 26-fortified breakfast cereal flakes. They see how the fe component of this heterogeneous mixture (cereal) retains its properties and can thus exist separated by physical means.
Vocabulary/Definitions
compound: A pure chemic substance consisting of two or more unlike chemical elements.
element: A substance consisting one type of atom.
heterogeneous mixture: A mixture that consists of visibly different substances or phases.
homogeneous mixture: A mixture that has the same uniform appearance and composition throughout its mass.
mixture: A substance consisting mixing two or more material.
solute: Material dissolved in a solution.
solution: A homogeneous mixture equanimous of 2 or more than substances.
solvent: A liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute.
Assessment
Post-Lesson Test: Administer the Mixtures Test to approximate educatee comprehension of the lesson content and concepts.
Lesson Extension Activities
Explain to students the distillation process for rough oil. Refer to the following source of data on the topic:
- How Oil Refining from Crude Oil Works http://science.howstuffworks.com/environmental/free energy/oil-refining2.htm
References
Humphrey, J. L. and Yard. E. Keller 2. Separation Process Engineering. New York, NY: McGraw-Hill, 1997. (Industrially oriented volume that includes performance, selection and calibration up data)
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Academy of HoustonContributors
Parnia Mohammadi; Roberto DimaliwatSupporting Program
National Scientific discipline Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was adult by the University of Houston'south Higher of Engineering under National Scientific discipline Foundation GK-12 grant number DGE-0840889. All the same, these contents do not necessarily represent the policies of the NSF and you lot should not assume endorsement past the federal government.
Last modified: February two, 2022
williamssuccionoth.blogspot.com
Source: https://www.teachengineering.org/lessons/view/uoh_sep_mixtures_less1
0 Response to "Can a Mixture Be Separated by Physical Means"
إرسال تعليق